UF Engineer Dr. Z. Hugh Fan leads team to make Zika virus detection faster and cheaper.
The adult Aedes aegypti mosquito’s body extends about half the width of a person’s knuckle. It may be small, but its feeding habits can have a big impact on human populations in warm parts of the globe. When an infected mosquito bites a person, it can transmit the Zika virus to him or her. To protect people from serious consequences of Zika virus infection and the potential contamination of blood and tissue products used in transfusions and medical procedures, testing patients and screening donors has become essential.
Generally, symptoms of Zika disease are mild, including fever, rash, conjunctivitis, muscle and joint pain, malaise, and headache, which usually last for 2–7 days. The World Health Organization (WHO) reports that the majority (about 80%) of people infected with Zika virus do not develop any symptoms. However, in October 2015, Brazil reported an association between Zika virus infection and microcephaly and other congenital anomalies in fetuses and newborns. Infection with the virus was also found to sometimes lead to Guillain-Barré syndrome, a condition in which the immune system attacks parts of the peripheral nervous system.
In the U.S., the Centers for Disease Control and Protection (CDC) worked quickly with academic and industry partners to develop diagnostic tests that could identify whether or not a person is actively infected by the virus, or had previously been exposed to the virus. Among the tests developed were a nucleic acid test (NAT), which detects the presence of Zika virus RNA in a specimen, and an enzyme-linked immunoassay (ELISA) test for the presence of anti-Zika virus antibodies that are produced by those infected with the virus. Both types of tests are currently performed at CDC labs, at CDC-qualified testing laboratories, in some hospital diagnostic microbiology laboratories, and at Public Health Labs. Special equipment is required to run the NAT and ELISA tests, and results can take up to three weeks to be reported to the attending physician.
UF researchers, with funding from the state of Florida, have developed a rapid, cost-effective point-of-care test for the Zika virus that can be used in the field.
Dr. Z. Hugh Fan, professor and George N. Sandor Faculty Fellow at the Herbert Wertheim College of Engineering’s Department of Mechanical and Aerospace Engineering, and Dr. John Lednicky, Research Professor at the College of Public Health and Health Professions’ Department of Environmental and Global Health, led a team that developed a miniaturized device for analysis of the virus in blood, saliva, and urine specimens. Using the device, they demonstrated the reproducible detection of Zika virus.
Their device features innovative ball-based valves that enable the storage and sequential delivery of reagents, a mixing unit where the reaction (virus lysis) takes place, and a paper-based detection unit that allows for detection of the results by the naked eye or with the use of a cellphone camera.
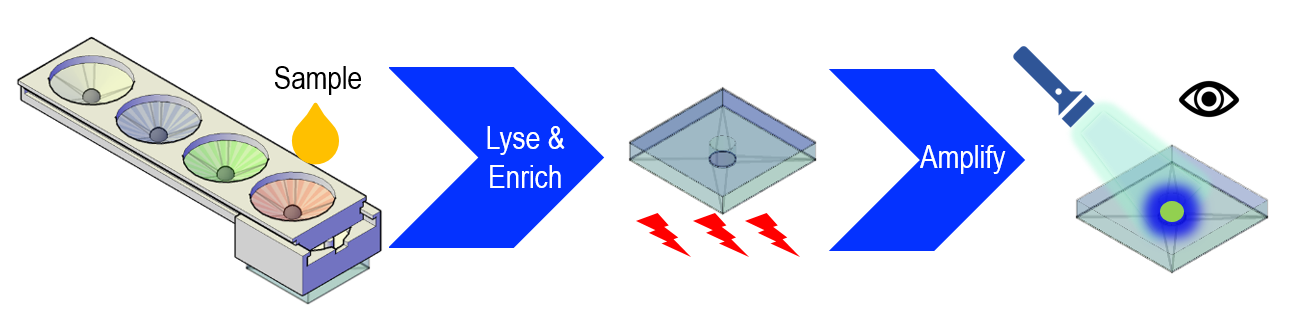
Dr. Fan commented on the test method, “We use an isothermal nucleic acid amplification method rather than (the) often-used polymerase chain reactions (PCR) so that thermal management is simpler.” The paper unit is placed in a commercially available coffee mug that provides a constant temperature for the development of the resulting color.
Because fever due to Zika virus is not specific, (countless viruses induce fever) and an infection by the virus can be misdiagnosed, it is important to have a point-of-care testing platform to accurately and quickly identify Zika virus infection for patient management and clinical diagnostics. In addition, because Zika virus generally causes mild or no symptoms, affected patients in an outbreak area may not seek medical care. The virus can then be transmitted through other non-vector-borne transmissions, including sexual activities and blood transfusion. A point-of-care testing platform could be useful for screening asymptomatic patients to prevent possible Zika virus transmission, as well as for safeguarding the blood supply at blood-donation sites.
Dr. Fan and the team have published the results of their current research in a high-impact journal (Angewandte Chemie International Edition), and UF has filed for a patent on the technology. The next step for Dr. Fan and Dr. Lednicky will be to do a large-sample validation test using their device.
Aedes aegypti was first identified as a carrier of the Zika virus in humans in 1952. According to the WHO, “From the 1960s to 1980s, rare sporadic cases of human infections were found across Africa and Asia, typically accompanied by mild illness.” The first recognized outbreak occurred in Micronesia in 2007. A large outbreak occurred in French Polynesia in 2013. Then, as the 2016 Olympics approached, Brazil, the hosting country, experienced a large outbreak in 2015. Further outbreaks and evidence of transmission soon appeared throughout the Americas, Africa, and other regions of the world. To date, a total of 86 countries and territories have reported evidence of mosquito-transmitted Zika virus infection.

